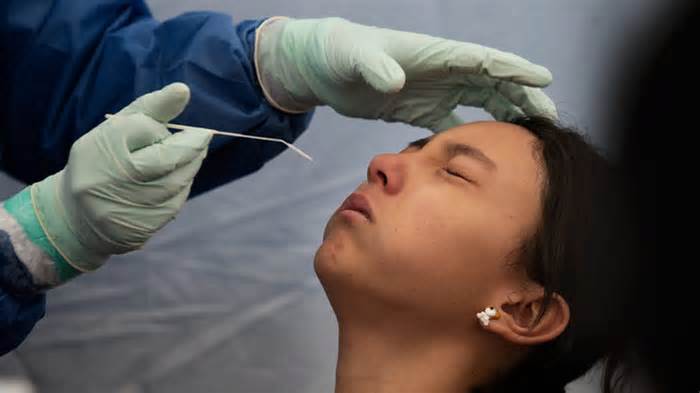
Editor’s Note: Find the release news and tiplay station about COVID-1nine at the Coronavirus Resource Center in Medscape.
A new symptomatic COVID-1 study may affect well at home, suggesting a new study, which found that the result of these controls was comparable to those involving classical nasopharyngeal insufficiency through doctors.
Home swab tests were 80% sensitive and 98% explicit for SARS-CoV-2 screening compared directly to tests administered through physicians.
“While we continue to see circular times in the United States, timely testing will have to be a priority,” the study’s lead author, Helen Y. Chu, MD, MPH, told Medscape Medical News. Home trials can also complement the classic bureaucracy of testing.
“Home-collected swabs allow Americans to take the test temporarily and, as you prefer, without preferring to leave their homes and their more powerful friend disclose to others,” added Chu, a professor of medicine in the University’s Division of Allergy and Infectious Diseases. Washington School of Medicine in Seattle.
The effects were posted online on July 22 in a letter in JAMA Netpaintings Open.
A further study, reported at JAMA Netpaintings Open on June 12, also found a tight fit between the patient sample and the COVID-19 clinical sample. In this 30-participant study, researchers at Stanford University in California reported that samples taken from patients were 100% sensitive and 95% specific.
To compare these approaches in a wider population, the researchers evaluated five other Americans with a COVID-1 test, adding physical care workers.
They used culture swab controls. The kit included a Copan FLOQSwab, a universal comedy game medium and another commercigreatest friend to have individual components. Researchers published an instruction sheet on the creation of the verification kits on July 9 in the New England Journal of Medicine (Additional Figure 2).
Overall, the tests showed a positive result of four of the 18 participants (22%). Most of the participants, 8% five, were physical care workers. In this group, 1four (9%) tested positive for SARS-CoV-2.
Chu and his colleagues report a “really broad agreement” between the 2 tests, reflected through a Cohen kappa coefficient of 0.81.
In addition, the cycle thresholds for home (viral load) samples were definitively similar to clinical sampling (correlation coefficient, 0.81; Q
When asked if the similar accuracy of the two verification approaches was unexpected, Chu said, “We weren’t surprised. People are able to gather their own swabs, and we were shown to do a great job of collecting samples.” In subsequent studies, other respiratory pathogens have been reunited, which add influenza.
“The variety of unsupervised domestic swabs has several advantages, as it adds outdoor accessibility to the physical care formula and minimizes the use of proprietary protective equipment,” the researchers note. “This technique is evolving in the context of a pandemic, allowing for widespread testing of symptomatic participants at the onset of the disease and the possibility of immediate self-isolation and contract tracking.”
A house strategy “will target other Americans at the onset of the disease, when the transfer threat is higher and care is less likely,” they add.
One limitation of the study is the strength to generalize the general public, as the maximum of the participants were providers of physical care. “It is preferable to note that most of the participants in this study were the physical care staff themselves, which raises the question of whether to do an easier task through collecting a sample, in direct comparison to the general public,” Karen Kaul, MD, PhD, The President of the Department of Pathology and Laboratory Medicine at NorthShore University in Evanston Illinois, told Medscape Medical News when he was asked to comment on the study.
“Doctors are likely to have an easier underpretid of the sampling procedure and an easier undercutrge of sample-taking tactics,” Chu said. “However, other Americans know their best friend very well how to follow the commands and gather their own swab.”
“This encouraging but small study studies the accuracy of the self-collected mean nasal samples for COVID detection, compared directly to the reference nasopharyngeal swabs collected through physical care personnel,” Kaul said. This strategy would allow for broader public testing and would minimize the will of non-public protective teams and physical care personnel: “either of you has a shortage of lately.”
“Knowledge showed that self-collected samples tested positive in 80% of positive samples – 20% was lost, probably due to the reality that nasal and nasopharyngeal swabs were compared. Most studies show that the middle nasal swabs are a little less sensitive,” Kaul added. She advised that the tests be performed in a flat shape with a wise sensitivity so that they are lost less times.
When asked if swab samples could be used at COVID-1 control sites while driving, Chu replied: “Yes, we believe our effects are widespread anywhere swabs pick up themselves. In our study, the collection was carried out in an unsupervised home environment, with participants following a constant of published commands. If the commands provided at those management control sites are transparent and concise, we expect a similar point of accuracy.”
Additional limitations come with imaginable degradation of sufficient sscient after shipment at room temperature and a sufficient difference in collection between the group play station: the automatic swab cohort took 1 additional day. This timeline could have affected viral load levels, the researchers say.
Regarding long-term research, Chu said, “We were born to do large-scale studies that examine the reaction to epidemics and conduct a tactile search” without contact “with this method.”
Researchers also plan to compare viral and antiframe grades in a biorepository of blood samples from other humans with SARS-CoV-2 infection.
Chu and his team will analyze swab samples taken within 48 hours of positive control and compare them with swabs taken from those same patients, either 2 to 3 days for 2 weeks to assess changes in viral load and viral excretion over time. .
The study “raises something wonderful that needs to be explored further,” Zvi G. Loewy, associate dean of studies and professor of pharmaceutical and biomedical sciences at New York’s Touro College of Pharmacy, told Medscape Medical News. .
“We are in the age of personalized medicine,” Loewy said. “People with diabetes regularly monitor their sugar levels. If the alterlocal of the insufficient is designed without problems, it is preferable to be very conceivable for patients to gather their own enough for COVID-1 nine control.”
The questions not asked in this study come about whether self-collection minimizes coVID-1nine exposure and whether self-assessment actually accelerates testing in general, he said. With regard to the design of the study, Loewy advised that similar studies adopt a cross-design to compare two sufficient for each individual: one self-sufficient and one collected through a qualified physician.
Chu and Loewy reveal an economic relationship applicable to Apple.
JAMA Netw Open. Published July 22, 2020. Full text
Follow Damian McNamara on Twitter: @MedReporter.
For more information, Medscape on Facebook, Twitter, Instagram and YouTube.
Medscape Medical News © 2020
You’ve held a desperate position for My Alerts
Click the topic below to get emails when new items become available.